Chemistry is a subject loaded with complex words and phrases. This might seem a little overwhelming. Getting to grips with all the scientific vocabulary might be the thing that is putting you off opening your textbooks in the first place. Luckily, with this handy guide to the most important general chemistry terms, you should start feeling more comfortable quickly. They are worth knowing not only for chemists themselves, or for the professors and engineers who use them.
Instead, everyone should know these words and definitions, as they help to unlock that world of fizzing experiments, laboratories, and flaming reactions that is chemistry. So, if you are studying for exams, take a look at this introductory chemistry dictionary and learn something! If you know it already, check out our other articles with everything you need to know about chemistry.

Fundamental Terms in Chemistry: The Small Stuff
Let’s start small. Chemistry is the study of atoms, elements, compounds, and molecules. These are four terms to get you going. But what do they mean? It’s an important question, as these four things (and the stuff of which they are made) make up everything in the universe.
Every molecule is made up of atoms, which are held together by chemical bonds that define their structure and properties
The smallest unit of an element that retains its chemical properties.
An element – iron, say, or oxygen – is a pure substance or something that you cannot break down into another substance. You can only break it down into atoms or the smallest bits of the element you can still recognise as this or that element. An element is only lots of the same atom. How about molecules and compounds? These are slightly different.
Molecules
Two (or more) atoms joined – or bonded – together. So, whilst an oxygen atom is not a molecule, if two oxygen atoms bond together, then that is a molecule (what we would call O²).
Compounds
Molecules that have bonds between two different types of atoms. In this case, if you add a carbon atom to O², you get CO². This is carbon dioxide, and this is a compound.
Accuracy
A measure of how close a measured value is to the true or accepted value. This is important in chemistry experiments to ensure reliable results.
We've also published a piece on wicked chemistry facts if you're interested. Check it out!
Even Smaller: Further Terms in Basic Chemistry
We need to go smaller still to understand how these atoms bond together. As you may know from your chemistry lessons, atoms are made of particles, which either clump in the atom’s nucleus or spin around that nucleus.
These particles have a charge that is either positive, neutral, or negative. The nucleus (the centre of the atom) holds the protons, which have a positive charge, and the neutrons, which are neutral. The negatively charged electrons, meanwhile, orbit the nucleus.
Electrons play a crucial role in bonding, whether in covalent bonds where atoms share electrons, or in ionic bonds where ions are formed through the transfer of electrons. The process of ion formation is key in acids and bases, as acids release hydrogen ions (H⁺), while bases accept them
These are key to understanding how molecules and compounds are made. Atoms bond because of these electrons, and there are two types of bonds: ionic and covalent.
Covalent bonds
Two atoms share a pair (or more than one pair) of electrons.
Ionic bonds
One atom donates an electron to another. When this happens, the donating atom becomes an ion: it becomes positively charged. Metals are those elements that like to lose electrons, forming bonds and developing positive charges.
Oxidation Number
A number assigned to an element in a chemical compound that represents the number of electrons lost or gained in bonding.

Start taking chemistry courses here.
Covalent Bonds: Atoms share electrons.
Ionic Bonds: One atom donates an electron, creating charged ions.

Chemistry’s Key Vocab: Chemical States and Compounds
Now that we’ve covered the basic chemistry terms, let’s look at some words you’ll hear flying around your chemistry department. Molecules make up substances, which can be found in three different states. You will probably have heard these already, but it is important to remember that a substance can change its state due to heat and pressure.
Water is an essential liquid in chemistry, often acting as a solvent in solutions where molecular compounds dissolve based on their concentration and chemical properties.
Gases
These are substances with no fixed shape or definable volume.
Liquids
Fluid substances, with no fixed shape but with a definite volume.
Solid
These substances are more stable, with their molecules more tightly packed. They have a more fixed shape, and a definite volume.
Substances can be pure elements, compounds, or mixtures. In chemistry, a mixture is defined as a substance made of two or more combined elements but not chemically bonded like a compound. There are different types of compounds, some of which most basic chemistry courses will require you to know:
Hydrocarbons
These organic compounds contain – as the term suggests – only hydrogen and carbon.
Polymers
Large molecules – either naturally occurring or synthetic and produced in a lab – that are formed of lots of bonded smaller molecules (often hydrocarbons).
Salt
An ionic compound whose charge is neutralised. It combines ions with a positive charge with those of a negative one.
Aqueous Solution
A solution in which water is the solvent, making up a large portion of chemical reactions in biological and industrial processes.
Solubility
The ability of a substance to dissolve in a solvent, determining how well substances mix.
Alloy
A mixture of two or more elements, where at least one is a metal, resulting in enhanced material properties such as strength and corrosion resistance.
Finally, in this section, we have acids and alkalis. These are opposites. Acids contain hydrogen, donate protons and make positive ions in water. Alkalis produce negative ions in water. You’ll see this again below, but if you want something a little more in-depth, try out our piece on the central concepts in chemistry. Check for a good chemistry tutor on Superprof.

Essential Terminology for Chemical Processes and Reactions
For most chemistry courses, you will need to know some basic terms for chemical reactions – or you will never understand what happens in the laboratory or an experiment! Firstly, you need to know the three terms of a chemical reaction. These are…
Reactant
The substance which is present at the start of the reaction.
Catalyst
The substance that enables the reaction, but that isn’t changed by it.
Product
What you get at the end of the reaction.
Step 1
Reactants
The starting substances
Step 2
Catalyst
Speeds up the reaction (if applicable)
Step 3
Reaction Occurs
Bonds break and reform
Step 4
Products
The final substances
Activation Energy
The minimum energy required to initiate a chemical reaction.
Redox Reaction
A reaction involving the transfer of electrons between two species, encompassing both oxidation and reduction.
Oxidation: Loss of electrons
Reduction: Gain of electrons
Example: Rusting of iron (Fe → Fe³⁺)
Precipitate
An insoluble solid that emerges from a liquid solution during a chemical reaction.
Endothermic Reaction
A reaction that absorbs heat from its surroundings, making the environment feel cooler.
Exothermic Reaction
A reaction that releases heat into its surroundings, often producing light or heat.
All reactions are either endothermic or exothermic, meaning they either take in energy or give it out. In this table, you can find some important words for the main types of reactions you will be dealing with:
| Oxidation | A reaction, usually involving oxygen, in which an electron is lost. |
|---|---|
| Reduction | When electrons are added to an atom (the opposite of the above!) |
| Distillation | When a mixture loses a liquid by evaporation and condensation. |
| Thermal Decomposition | Breaking a compound into two or more substances by heating. |
| Titration | If you know the concentration of a solution, you can use titration to determine the concentration of a different solution. |
Read some fun chemistry facts here!
The Language of Chemistry: Measurements, and the Periodic Table
Understanding the textbook terms used in your chemistry course is not only about knowing the atomic structures of states of matter. You also need to know the ways in which a chemist might make a calculation or measure a given substance. This indispensable terminology will help in any chemistry class.
The Periodic Table
You’ll have noticed this in any chemistry lab you’ve seen. This is the table of the elements, arranged in order of atomic number. It was invented by a bloke called Mendeleev, about whom you can learn more in our piece on the most important chemists ever.
Atomic number
An atom’s number of protons – and, therefore, electrons, as they are equal.
Mass number
The number of protons plus the number of neutrons.
Transition element
Elements in groups three to twelve of the periodic table. Also known as transition metals.
Mole
The unit used to identify a given amount of a substance. A mole of any substance contains the same number of atoms as a mole of another substance.
The number of particles in one mole of a substance!
Reactivity
How reactive a substance is in relation to another. You get a reactivity series if you put substances in order of relative reactivity.
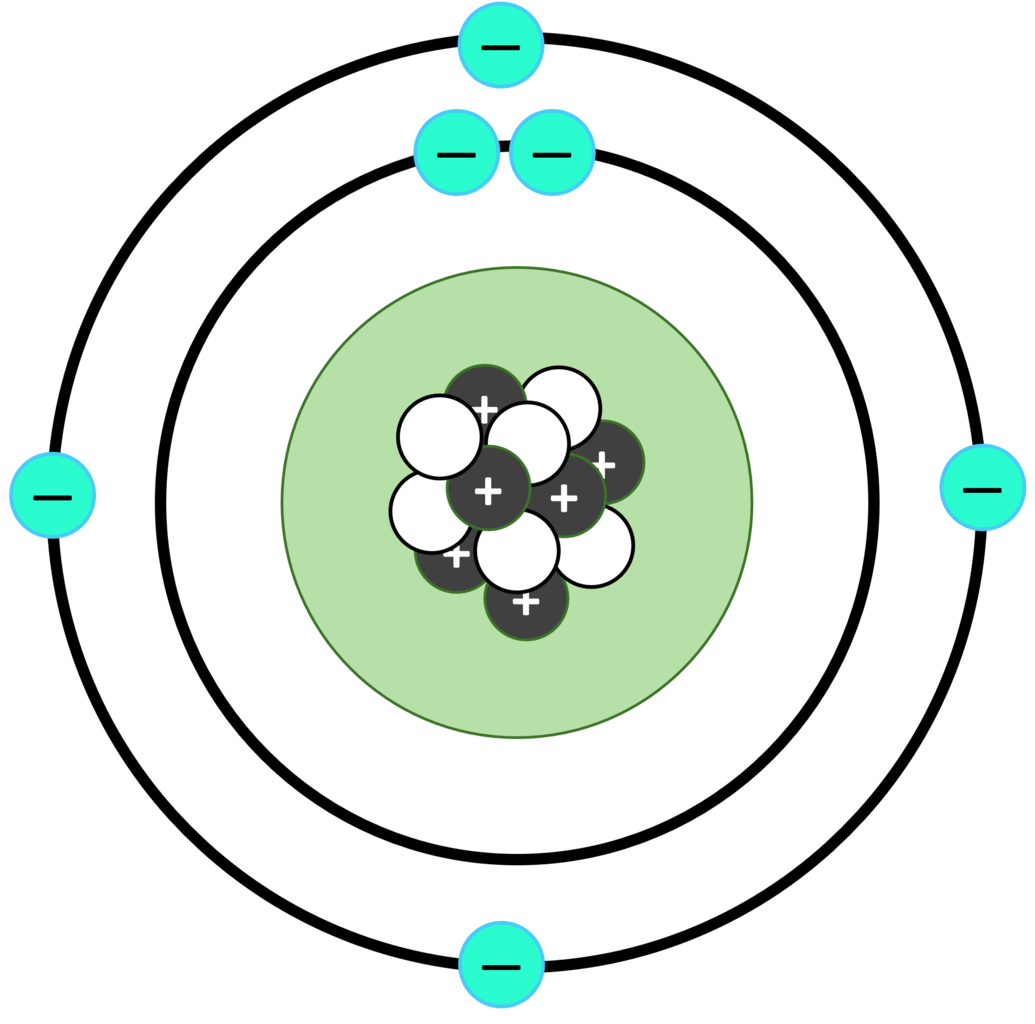
Carbon
- Symbol: C
- Atomic Number: 6
- State at Room Temp: Solid
- Uses: Found in all living things, fuels, and plastics
Alongside a reaction, you will probably need to write a chemical equation. This shows, in written form, what reactants are involved and what products are produced.
You will also need to know another scale, the pH scale. This is used to describe how acidic or alkaline a substance is. It ranges from 0 to 14, with the most acidic having the lowest number and the most alkali having the highest. Neutral substances are pH7.
Find a VCE chemistry tutor today on Superprof.
A Word on the Most Important Chemistry Equipment.
Any introduction to chemistry vocabulary would be lacking without a mention of the most essential equipment any scientist might use in their labs. Chemistry is theoretical and analytical, empirical and therefore practical too! Check out more in our article on the basic chemistry kit.

Bunsen burner
Using this will be one of the highlights of your high school chemistry experience. By plugging this into a gas tap, you will get heat and a flame for your chemistry experiments.
Tripod and gauze
Over a Bunsen burner can be placed a stand that can hold beakers to be filled with chemical elements and solutions.
Test tube
The iconic tool of chemical science, this is a slim tube in which you will keep and perform experiments on your solutions. A boiling tube is a larger variety of test tube, in which – you guessed it! – you can boil things.
Burettes
Like test tubes, they have measurements and are clamped – so that you can drip little bits of solution. They are used mainly for titrations.
This is used not only in chemistry but in biology and medicinal science, too. A little squeezy plastic tube to transport liquids.
Remember that chemistry affects us every single day. Why not read up on some life-changing chemistry discoveries while you're here? Find a chemistry tutor here.
Summarise with AI: